NIH New Data Management and Sharing Requirements
The new National Institutes of Health (NIH) Data Management & Sharing (DM&S) Policy supersedes the 2003 requirements and will become effective on January 25, 2023. This policy aims to make research data management and sharing a norm while maximizing the availability and reusability of research data to foster trust and transparency in science.
Essentially, the new NIH policy requires researchers seeking funding to:
- Submit a DM&S Plan (equivalent to DMPs for other funding agencies) outlining how scientific data and any accompanying metadata will be managed and shared, considering any potential restrictions or limitations that may apply to your project.
- Comply with the Data Management and Sharing plan approved by the funding Institute or Center within the NIH.
This new policy applies to research, funded or conducted in whole or partly by NIH, that generates scientific data. It includes research funded or conducted by extramural grants, contracts, intramural research projects, or other funding agreements regardless of NIH funding level or funding mechanism. The DM&S Policy does not apply to research and other activities that do not generate scientific data, including training, infrastructure development, and non-research activities.
Not sure which policy applies to your research? When in doubt, use NIH's decision tool to determine which policies apply to your research.
The UCSB's Library Research Data Services department can help you navigate this new policy, connect with campus resources and conform to the NIH requirements.
For questions and requests, email: rds@library.ucsb.edu
Recommended Resources
- NIH - Scientific Data Sharing: Official NIH website with policies, guidelines, events, and more.
- Data Management & Sharing Policy Overview: Learn what is expected from investigators and institutions under the 2003 NIH Data Sharing Policy.
- Glossary: Data Terms related to the NIH DMS Plan and Policy
- Final NIH Policy for Data Management and Sharing: NIH Final Policy [NOT-OD-21-013] & Related Announcements
- FAIR Principles: The FAIR Guiding Principles for scientific data management and stewardship
- FAIR Principles (Handout): Data Literacy Series on FAIR data.
- Allowable Costs for Data Management and Sharing: Categories of allowable NIH costs associated with data management and sharing.
Plan Ahead
In preparation for the new policy, we suggest you follow these seven high-level steps and explore the links below:
- Determine your project timeline. If you have an active NIH award seeking renewal in 2023 or are planning to submit a new NIH proposal this year, developing a DM&S Plan should be a high priority. This timeline is critical if you work with external collaborators, as it may take time to set up appropriate data procedures and agreements.
- Familiarize yourself with the FAIR principles which guided the creation of this new policy. The language around these four principles can help you develop your DM&S Plan.
- Get familiar with the new policy and requirements, including the supplemental information and notes.
- Assess your data management practices relative to the policy, especially around documenting existing practices and developing new ones to address the increased emphasis on data sharing and administrative oversight.
- Reach out to the Library and the Office of Research for available campus resources (e.g., computing, de-identification, storage, consulting). We can help you assess the options available and connect with relevant departments.
- Identify and budget for external resources and labor for data-related activities (e.g., cleaning/wrangling, documentation, preservation).
- Draft your DM&S Plan and request experts' review. The UCSB's Library Research Data Services department (rds@library.ucsb.edu) can review and provide feedback on your plan before submission.
Elements of a DM&S Plan
According to the policy, a DM&S Plan should include the following sections:
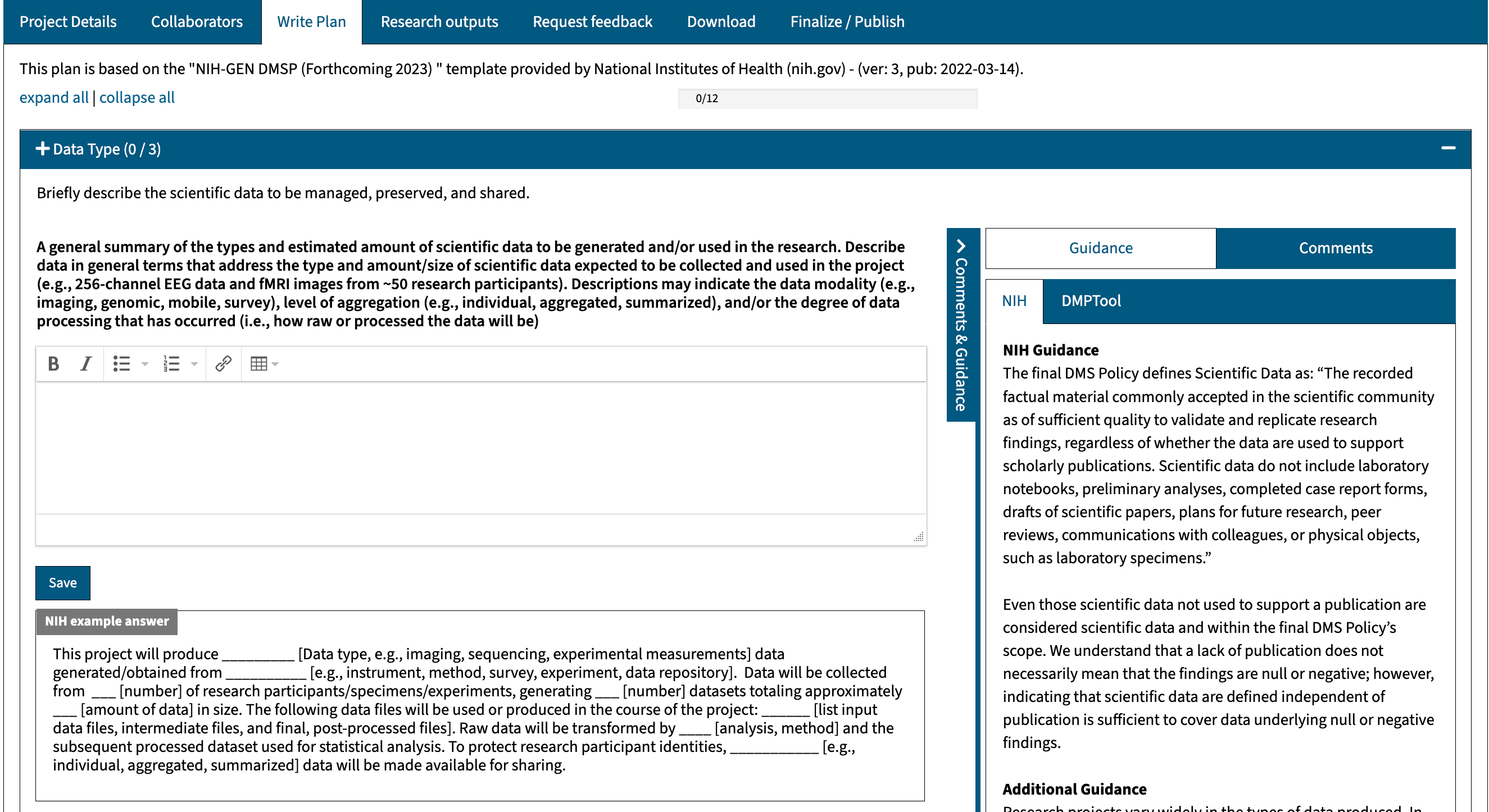
- Data Type: describe the scientific data to be managed, preserved, and shared.
- Related Tools, Software and/or Code: indicate whether specialized tools are needed to access or manipulate shared scientific data to support replication or reuse and the names of the required tools and software.
- Standards: provide what standards will be applied to the scientific data and associated metadata (i.e., data formats, data dictionaries, data identifiers, definitions, unique identifiers, and other data documentation).
- Data Preservation, Access, and Associated Timelines: outline plans and timelines for data preservation and access.
- Access, Distribution, or Reuse Considerations: indicate plans to maximize the appropriate sharing of scientific data generated from NIH-funded or conducted research, consistent with privacy, security, informed consent, and proprietary issues.
- Oversight of Data Management and Sharing: demonstrate how compliance with the plan will be monitored and managed, with what frequency, and by whom (e.g., titles, roles, responsibilities).
These six sections or basic elements of a DM&S Plan can be mapped into 10 rules which can maximize the NIH recommendations:

Source: Gonzales S., Carson M. B., Holmes K. (2022). Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan. PLoS Comput Biol 18(8): e1010397. https://doi.org/10.1371/journal.pcbi.1010397
Recommended Resources
Elements of an NIH Data Management and Sharing Plan
[NOT-OD-21-014] Supplemental Information to the NIH Policy for Data Management and Sharing.
Data Management and Sharing Plan Checklist
Built-in guidance for the upcoming policy.
Data Literacy Series handout on the advantages of using the DMPTool.
A complete and vetted example based on the NIH 2023 requirements.
Developed by the NIH DMSP Guidance Working Group
A complete and vetted plan following the NIH's 2023 requirements.
Write your DM&S Plan

For a template with specific guidance and essential tips (including boilerplate language), we suggest you create your NIH DM&S Plan using the DMPTool. The DMPTool is a free system that enables researchers to develop machine-actionable plans aligned with the most up-to-date funder requirements. Get started by following the steps below:
Through the built-in feedback feature, you may request our team to review your plan and provide comments before the submission deadline.
Choose a Repository
NIH encourages researchers to select data repositories that exemplify the desired characteristics to ensure that data are managed and shared in ways that are consistent with FAIR data principles as expressed in the Supplemental Information to the NIH Policy for Data Management and Sharing: Selecting a Repository for Data Resulting from NIH-Supported Research [NOT-OD-21-016].
Dryad, supported by the UC and available free of charge for affiliates, is listed among NIH's recommended repositories. Please consult the FAQs or this handout to learn more about Dryad's capabilities. If Dryad is not the best solution to house your project data, our team can help you identify a more appropriate repository to meet the NIH requirements. To schedule a demo or request data archiving assistance, don't hesitate to get in touch with rds@library.ucsb.edu.
FAQs
The NIH policy does not determine specific requirements or conditions for data sharing. When you share your data, you should address the NIH’s goal of making data as accessible as possible and follow the practice “as open as possible, as restricted as needed.”
NIH expects all shareable data to be made available, whether associated with a publication or not. All data used or generated as part of a grant must be managed, but not all data should be shared. You should not share data if doing so would violate privacy protections or applicable laws. You may share data related to human subjects, but your plan should address how data sharing will be communicated in the informed consent process (e.g., consent forms, waivers of the consent). Before submitting your data to your chosen repository, you will need to: Bundle your data together in logical “chunks” for citation and reuse. Appropriate bundling makes it easy to assign a persistent identifier(s) (e.g., DOI) to the dataset. These identifiers, usually assigned by data repositories, make it easier for others to cite your data and for the NIH to track compliance.
When preparing your data for sharing, consider these steps:
-
De-identify your data, if needed. Remember to remove both direct and indirect identifiers from your dataset.
-
Convert your data to an open, machine-readable file format whenever possible.
-
Use data and metadata standards appropriate to your field (if any); consult fairsharing.org for standards.
-
Document your code, scripts, and workflows [tips].
-
Document the dataset thoroughly in a separate Readme.txt file (customizable template), and/or create metadata according to the format required by your chosen repository or discipline.
-
Prepare your project files for submission in an organized structure [tips].
-
Choose a certified and reliable repository to archive your data following NIH’s recommendations [NOT-OD-21-016].
-
Specify the license governing your data and code [tips].
You must share your project data when you publish your work or before your performance period ends, whichever comes first. In general, you should make your data accessible as soon as possible. You can also use relevant requirements and expectations, such as data repository policies, award record retention requirements, or journal policies to decide when to share your datasets.
-
NIH recommends sharing datasets through established data repositories to improve the data’s FAIRness (Findability, Accessibility, Interoperability, and Reusability). While NIH supports many data repositories, your data may not be appropriate for an NIH repository.
-
You should also consider data repositories supported by other public and private organizations and domain-specific and general repositories in case there are no disciplinary options. For more information, see Supplemental Information to the NIH Policy for Data Management and Sharing: Selecting a Repository for Data Resulting from NIH-Supported Research [NOT-OD-21-016].
Program staff at the proposed NIH Institute or Center will assess DMS Plans to ensure the Elements of a DM&S Plan have been adequately addressed and to determine the reasonableness of those responses. Applications selected for funding will only be funded if the DM&S Plan is complete and acceptable. Peer reviewers can comment on the proposed data management budget. If data sharing is integral to the funding opportunity, reviewers will evaluate the DM&S Plan and may factor that information into the score outlined in the evaluation criteria. More information about the assessment process is described in the Implementation Details for the NIH Data Management and Sharing Policy [NOT-OD-22-189].
If the DM&S Plan provided in the application cannot be approved based on the information provided, applicants will be notified that additional information is needed. This will occur through the Just-in-Time (JIT) process. Applicants will be expected to communicate with their Program Officer or Grants Management Specialist to resolve any issues that may prevent the plan’s approval and submit a revised version if required.
Following the award, recipients must comply with the version of the DM&S Plan approved by the funding NIH Institute or Center. Plans may be updated during regular reporting intervals as part of the annual Research Performance Progress Report (RPPR) process. The funding NIH Institute or Center must approve changes to the DM&S Plan.
List of questions related to the 2023 policy.